FDA Grants Novavax COVID-19 Vaccine Approval Under Specific Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Grants Novavax COVID-19 Vaccine Approval Under Specific Conditions
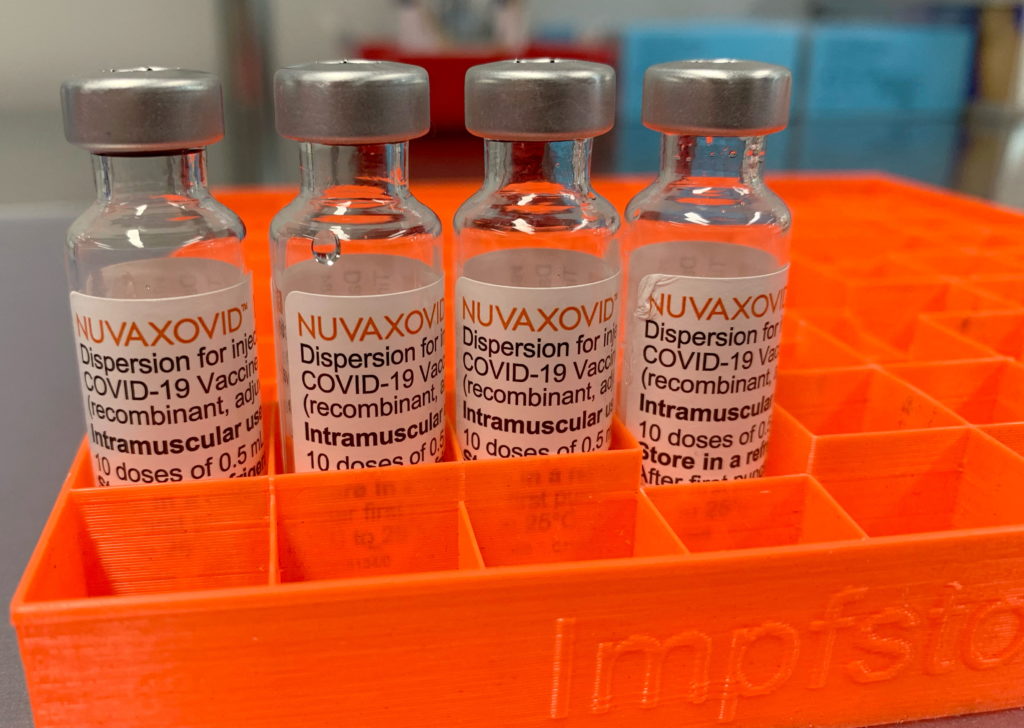
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant development in the ongoing fight against the pandemic. This protein-subunit vaccine, developed using a more traditional technology than the mRNA vaccines currently dominating the market, offers a new option for those hesitant about the newer approaches. However, the approval comes with specific conditions, highlighting the ongoing scrutiny and evolving understanding of COVID-19 vaccines.
A Different Approach to Vaccine Technology:
Unlike the Pfizer-BioNTech and Moderna vaccines, which utilize mRNA technology, Novavax's Nuvaxovid (its trade name) employs a protein-subunit approach. This involves using harmless pieces of the virus's protein to trigger an immune response. This traditional method may appeal to individuals who have concerns about the newer mRNA technologies. Many people are looking for vaccine options that use more familiar methods, and Novavax’s approval provides just that. This makes it a potential game-changer for vaccine hesitancy, offering a viable alternative for those seeking a different vaccination strategy.
Conditional Approval: What Does It Mean?
The FDA's conditional approval isn't a full-fledged license. It means the agency has determined that the vaccine's benefits outweigh its risks, based on available data. However, further studies and data collection are required to confirm long-term safety and efficacy. This conditional approval allows for the vaccine's immediate distribution but necessitates ongoing monitoring and data analysis by Novavax to maintain this authorization.
Specific Conditions for Continued Approval:
The FDA has outlined several specific conditions that Novavax must meet to retain the conditional approval. These include:
- Continued Safety Monitoring: Novavax is required to continue monitoring the vaccine's safety profile through robust post-market surveillance. This involves tracking adverse events and analyzing data to detect any unforeseen issues.
- Real-World Efficacy Data: Real-world data on the vaccine's efficacy against various COVID-19 variants is crucial for continued approval. Novavax needs to demonstrate the vaccine’s continued effectiveness against emerging variants.
- Manufacturing Compliance: Maintaining high standards of manufacturing is paramount. The FDA will continue to scrutinize Novavax's manufacturing processes to ensure consistent quality and safety.
Who Might Benefit from the Novavax Vaccine?
While the Novavax vaccine offers an alternative approach, it's essential to consult with a healthcare professional to determine if it's the right choice for you. Certain individuals might find this vaccine particularly appealing:
- Individuals hesitant about mRNA vaccines: The different technology might alleviate concerns about mRNA vaccines.
- Individuals with specific pre-existing conditions: While further research is needed, this vaccine could potentially be a suitable option for some individuals with specific health concerns. Always consult your doctor.
Looking Ahead: The Future of COVID-19 Vaccination
The FDA's conditional approval of the Novavax vaccine is a significant step forward. It diversifies the available COVID-19 vaccine options, potentially addressing vaccine hesitancy and increasing vaccination rates. However, the conditional nature of the approval underscores the importance of ongoing research and monitoring in the evolving landscape of COVID-19 vaccination. The availability of this alternative vaccine strategy is an important development and may influence public health strategies in the coming months.
Call to Action: Consult your doctor or healthcare provider to discuss which COVID-19 vaccine is best for you, considering your individual health history and preferences. Staying informed about COVID-19 vaccines is critical for making informed decisions regarding your health and the health of your community. For further information on COVID-19 vaccines, visit the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Novavax COVID-19 Vaccine Approval Under Specific Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 International Support Urged Bali Prioritizes Tourist Safety And Conduct
May 20, 2025
International Support Urged Bali Prioritizes Tourist Safety And Conduct
May 20, 2025 -
 The Return Of Star Wars Battlefront A Detailed Look
May 20, 2025
The Return Of Star Wars Battlefront A Detailed Look
May 20, 2025 -
 Global Support For President Biden Following His Cancer Diagnosis Announcement
May 20, 2025
Global Support For President Biden Following His Cancer Diagnosis Announcement
May 20, 2025 -
 The Putin Trump Dynamic Shifts A Power Play Analysis
May 20, 2025
The Putin Trump Dynamic Shifts A Power Play Analysis
May 20, 2025 -
 Jon Jones And Tom Aspinall Heated Exchange Ignites Ufc Controversy
May 20, 2025
Jon Jones And Tom Aspinall Heated Exchange Ignites Ufc Controversy
May 20, 2025
