FDA Grants Approval To Novavax COVID-19 Vaccine, But With Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
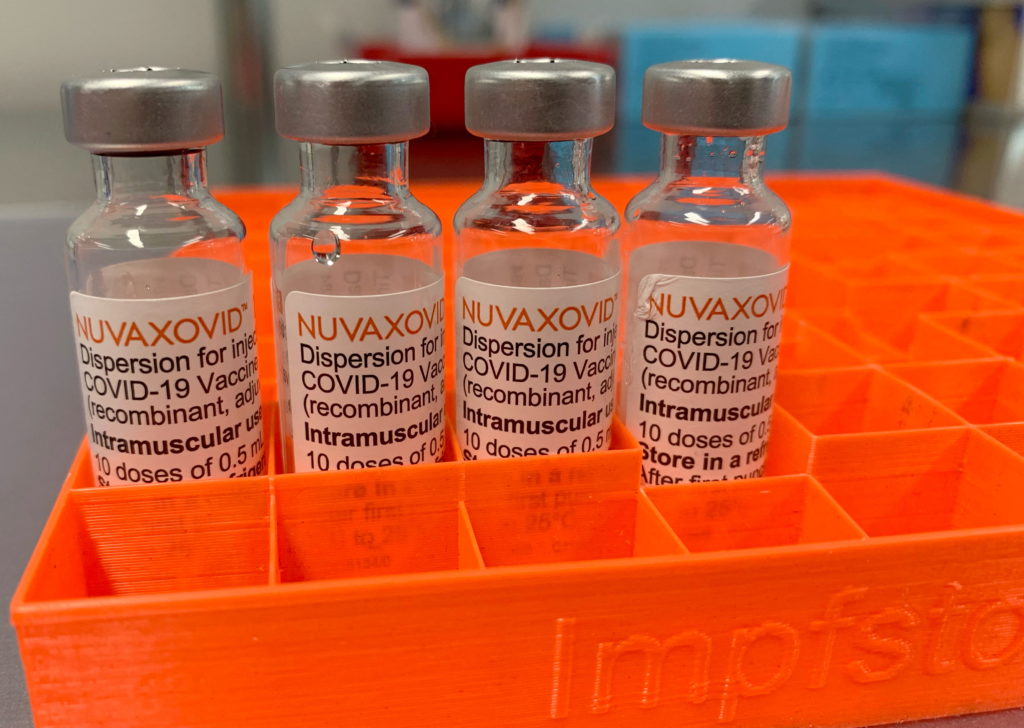
FDA Grants Approval to Novavax COVID-19 Vaccine, But with Conditions
Novavax's COVID-19 vaccine, Nuvaxovid, finally receives full FDA approval, but with specific limitations impacting its widespread rollout. This landmark decision, announced [insert date], marks a significant step for the pharmaceutical company, yet the attached conditions raise questions about the vaccine's immediate impact on the ongoing pandemic.
The approval, a much-anticipated event following months of review, grants Nuvaxovid full licensure for individuals 18 years of age and older. However, the FDA's approval comes with significant caveats. Unlike the widely used mRNA vaccines from Pfizer-BioNTech and Moderna, which received full approval much earlier, Nuvaxovid's approval is conditional on the company meeting specific post-market requirements.
This conditional approval hinges on Novavax fulfilling ongoing commitments to data collection and analysis. The FDA will closely monitor the vaccine's safety and efficacy profiles post-market, focusing particularly on [mention specific areas of concern the FDA cited]. This stringent oversight reflects the need to thoroughly assess long-term safety data, a process that was expedited for the initial emergency use authorizations of other COVID-19 vaccines.
<h3>What does this mean for the public?</h3>
While full FDA approval lends an air of increased confidence, the conditional nature of the approval significantly impacts the immediate accessibility of Nuvaxovid. The vaccine's availability may be limited initially, potentially impacting its widespread uptake. Healthcare providers will need to carefully consider the FDA's stipulations and patient suitability before administering the vaccine.
Furthermore, the existing landscape of COVID-19 vaccination complicates matters. With high vaccination rates already achieved through mRNA vaccines and other available options, the need for another vaccine, even with full FDA approval, might be perceived as less urgent by many.
<h3>Understanding the Nuvaxovid Vaccine</h3>
Nuvaxovid uses a different technology compared to the mRNA vaccines. It's a protein-subunit vaccine, employing a more traditional approach to vaccine development. This protein-based technology may appeal to individuals hesitant to receive mRNA vaccines, offering an alternative option. However, it’s important to note that all COVID-19 vaccines authorized for use in the US have undergone rigorous testing and have proven effective in preventing severe illness, hospitalization, and death.
<h3>Looking Ahead</h3>
The FDA's conditional approval of Nuvaxovid represents a complex scenario. While it provides a new option for vaccination, the limitations surrounding its approval warrant careful consideration. Further research and ongoing monitoring of the vaccine's safety and efficacy will be crucial in determining its long-term role in combating COVID-19.
The FDA's decision underscores the rigorous standards applied to vaccine approval. While full approval is a significant achievement for Novavax, the attached conditions demonstrate the agency's commitment to prioritizing public health and safety. This decision highlights the ongoing evolution of the COVID-19 vaccine landscape and the need for continued vigilance in monitoring vaccine safety and effectiveness.
For further information on the FDA's approval and the Nuvaxovid vaccine, please consult the FDA website [link to FDA website]. Stay updated with the latest information on COVID-19 vaccines from reliable sources such as the CDC [link to CDC website] and your healthcare provider.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Grants Approval To Novavax COVID-19 Vaccine, But With Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 206 Run Target For Lsg Marsh And Markrams Match Winning Innings For Srh
May 20, 2025
206 Run Target For Lsg Marsh And Markrams Match Winning Innings For Srh
May 20, 2025 -
 Major Solar Storm Causes Continent Wide Communications Outages
May 20, 2025
Major Solar Storm Causes Continent Wide Communications Outages
May 20, 2025 -
 Reserve Bank Of Australia Lowers Rates To Two Year Low Amid Easing Inflation
May 20, 2025
Reserve Bank Of Australia Lowers Rates To Two Year Low Amid Easing Inflation
May 20, 2025 -
 Jamie Lee Curtis On Maintaining Her Friendship With Lindsay Lohan A Post Freaky Friday Interview
May 20, 2025
Jamie Lee Curtis On Maintaining Her Friendship With Lindsay Lohan A Post Freaky Friday Interview
May 20, 2025 -
 U S Treasury Market Reaction Yields Fall On Feds 2025 Rate Cut Outlook
May 20, 2025
U S Treasury Market Reaction Yields Fall On Feds 2025 Rate Cut Outlook
May 20, 2025
