FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
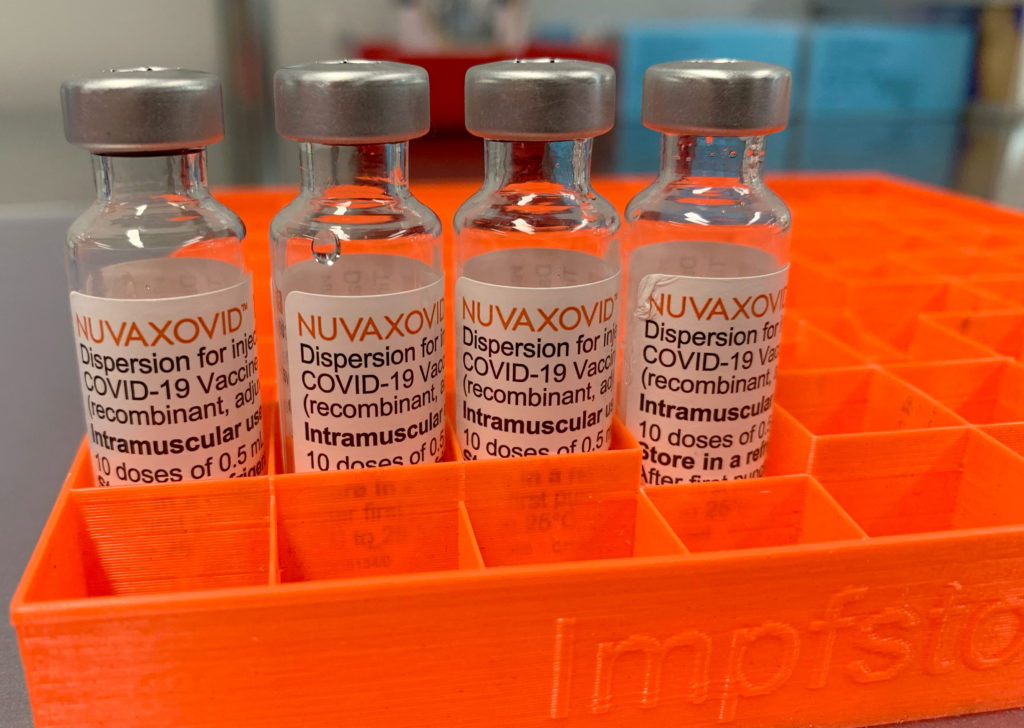
FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained
The FDA has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, offering a new option for individuals seeking vaccination. This protein-subunit vaccine represents a different technology compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, and this difference has implications for its use and potential advantages. However, understanding the FDA's use restrictions is crucial before considering this vaccine.
Understanding the Novavax Vaccine Technology
Unlike mRNA vaccines, which use messenger RNA to instruct cells to produce a viral protein, Novavax's Nuvaxovid uses a more traditional approach. It employs a protein-subunit technology, utilizing lab-grown fragments of the SARS-CoV-2 virus's spike protein. This protein is then combined with an adjuvant, a substance that boosts the immune response. This technology has been used in other vaccines for decades, potentially alleviating some concerns surrounding the newer mRNA technology. This familiarity may be a key factor for vaccine hesitant individuals.
FDA Approval and Use Restrictions:
The FDA's EUA for Nuvaxovid includes specific limitations. While available for individuals 18 and older, the vaccine is not yet authorized for younger age groups. This is primarily due to ongoing trials evaluating its safety and efficacy in adolescents and children. The FDA continues to monitor post-market safety data closely.
Who Should Consider the Novavax Vaccine?
The availability of Novavax presents a significant advantage for certain individuals:
- Those with mRNA vaccine hesitancy: The different technology may appeal to those hesitant about mRNA vaccines. This provides an alternative route to achieving COVID-19 immunity.
- Individuals with specific medical conditions: While not specifically indicated for any particular condition, the different mechanism of action may be beneficial for individuals with specific pre-existing conditions, though consultation with a physician is always recommended.
- People seeking a different vaccine experience: Some individuals may experience milder side effects with this vaccine compared to mRNA vaccines, though individual reactions vary.
What are the potential side effects?
Like all vaccines, Nuvaxovid can cause side effects. Common side effects reported in clinical trials include pain, redness, and swelling at the injection site, fatigue, headache, muscle aches, and joint pain. These side effects are generally mild and temporary. Severe side effects are rare.
The Importance of Vaccination:
Despite the introduction of new vaccines like Novavax, the importance of COVID-19 vaccination remains paramount. Vaccination continues to be one of the most effective strategies to reduce severe illness, hospitalization, and death from COVID-19. It’s crucial to stay up-to-date with booster shots as recommended by your physician and public health authorities.
Where to get the Novavax Vaccine:
The availability of the Novavax vaccine may vary depending on your location. Contact your local healthcare provider or check your state's health department website for information on vaccine availability and locations. [Link to CDC website for vaccine information].
Disclaimer: This article provides general information and should not be considered medical advice. Consult with your healthcare provider to determine the best vaccination strategy for your individual needs. The information provided here is based on currently available data and may be subject to change.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Use Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Bali Seeks International Help To Improve Tourist Behavior
May 20, 2025
Bali Seeks International Help To Improve Tourist Behavior
May 20, 2025 -
 Helldivers 2 Warbond Program Masters Of Ceremony Skins Arrive May 15th
May 20, 2025
Helldivers 2 Warbond Program Masters Of Ceremony Skins Arrive May 15th
May 20, 2025 -
 Bali Tightens Tourist Rules A Response To Growing Misconduct Concerns
May 20, 2025
Bali Tightens Tourist Rules A Response To Growing Misconduct Concerns
May 20, 2025 -
 Multi Year Contract Extension For Eagles Head Coach Nick Sirianni
May 20, 2025
Multi Year Contract Extension For Eagles Head Coach Nick Sirianni
May 20, 2025 -
 Jones Vs Ufc The Controversy Surrounding Tom Aspinalls Injury
May 20, 2025
Jones Vs Ufc The Controversy Surrounding Tom Aspinalls Injury
May 20, 2025
