FDA Approves Novavax COVID-19 Vaccine: Use Restricted
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
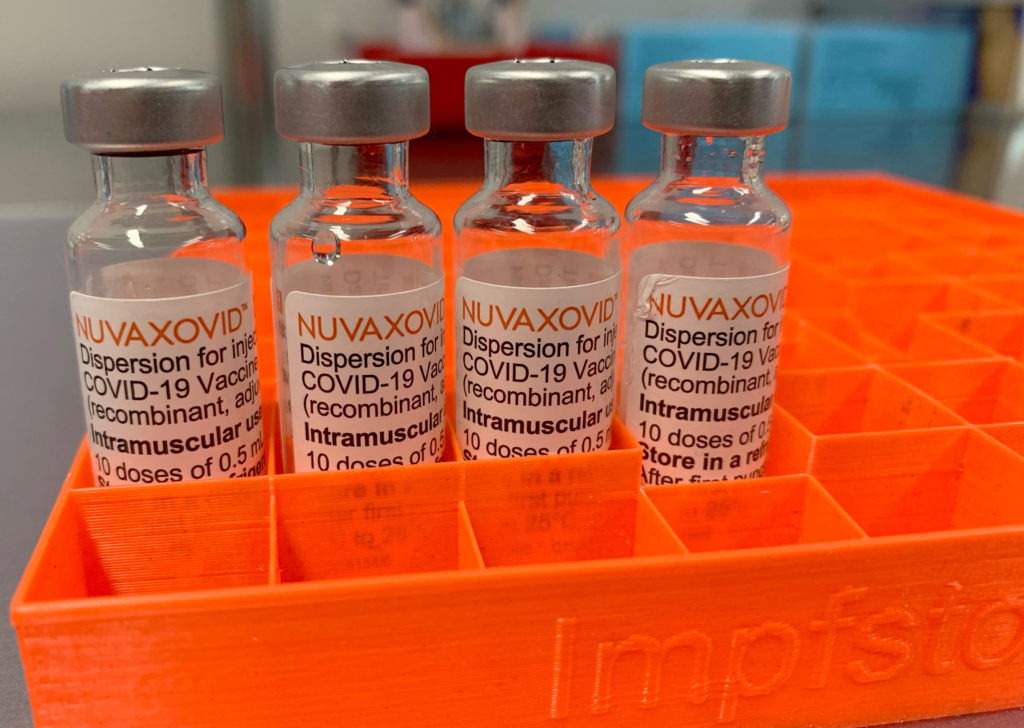
FDA Approves Novavax COVID-19 Vaccine, but with Restrictions
The FDA has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, but with a crucial caveat: its use is restricted. This announcement, while welcomed by some, presents a more nuanced picture than initial headlines might suggest. The approval comes after considerable delay and raises questions about the vaccine's role in the current pandemic landscape.
Understanding the Nuvaxovid Approval:
The FDA's approval of Nuvaxovid, a protein subunit vaccine, marks a significant milestone. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Novavax uses a more traditional approach, potentially appealing to individuals hesitant about the newer technologies. The vaccine's effectiveness against preventing severe illness, hospitalization, and death has been demonstrated in clinical trials. However, the restricted nature of the EUA is key.
Why the Restrictions?
The FDA's decision to grant only a restricted EUA stems from several factors. Firstly, the overall efficacy of Nuvaxovid against currently circulating COVID-19 variants is lower compared to other authorized vaccines. This means it might not provide the same level of protection against infection and transmission.
Secondly, the data supporting the vaccine's use in certain populations, particularly children and adolescents, is limited. Further research and trials are necessary before widespread rollout in these age groups can be considered.
Finally, the FDA emphasizes the importance of carefully considering the risk-benefit profile of Nuvaxovid in the current context. With other highly effective vaccines readily available, the agency's decision reflects a cautious approach.
Who Might Benefit from Nuvaxovid?
While the restricted EUA limits broad use, certain individuals may find Nuvaxovid a suitable option. These could include:
- Individuals with specific concerns about mRNA vaccines: The protein subunit technology might alleviate anxieties surrounding mRNA-based vaccines for some.
- Individuals who have previously had an adverse reaction to other COVID-19 vaccines: However, it's crucial to discuss this with a healthcare provider before opting for Nuvaxovid.
- Specific populations where further research might demonstrate greater benefit: Future studies could reveal particular demographics or clinical situations where Nuvaxovid offers a distinct advantage.
The Future of Nuvaxovid:
The FDA's approval is a step forward for Novavax, but the restricted nature of the EUA underlines the challenges the vaccine faces in a constantly evolving pandemic landscape. The company is likely to continue conducting research and submitting data to broaden the EUA's scope and address the current limitations. The long-term role of Nuvaxovid in COVID-19 vaccination strategies remains to be seen.
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine is a mixed bag. While offering a protein-subunit alternative, the restricted EUA highlights the necessity of carefully weighing its benefits against the availability of other, potentially more effective, vaccines. Further research will be crucial in determining its long-term place in the fight against COVID-19. Always consult with your healthcare provider to discuss which COVID-19 vaccine is right for you. For more information on COVID-19 vaccines, visit the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Use Restricted. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Ufc Fans React Jon Jones Hints At Retirement Amidst Aspinall Negotiation Delays
May 20, 2025
Ufc Fans React Jon Jones Hints At Retirement Amidst Aspinall Negotiation Delays
May 20, 2025 -
 Spains Airbnb Crackdown Thousands Of Holiday Rentals Suspended
May 20, 2025
Spains Airbnb Crackdown Thousands Of Holiday Rentals Suspended
May 20, 2025 -
 Stock Market Rally Continues S And P 500s Six Day Winning Streak Market Analysis
May 20, 2025
Stock Market Rally Continues S And P 500s Six Day Winning Streak Market Analysis
May 20, 2025 -
 Latonya Pottain My 600 Lb Life Star Dead At 40 Family Shares Details
May 20, 2025
Latonya Pottain My 600 Lb Life Star Dead At 40 Family Shares Details
May 20, 2025 -
 Semana De Carreras Del Real Madrid Preparacion Y Objetivos Clave
May 20, 2025
Semana De Carreras Del Real Madrid Preparacion Y Objetivos Clave
May 20, 2025
