FDA Approves Novavax COVID-19 Vaccine: Usage Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approves Novavax COVID-19 Vaccine: Usage Restrictions Explained
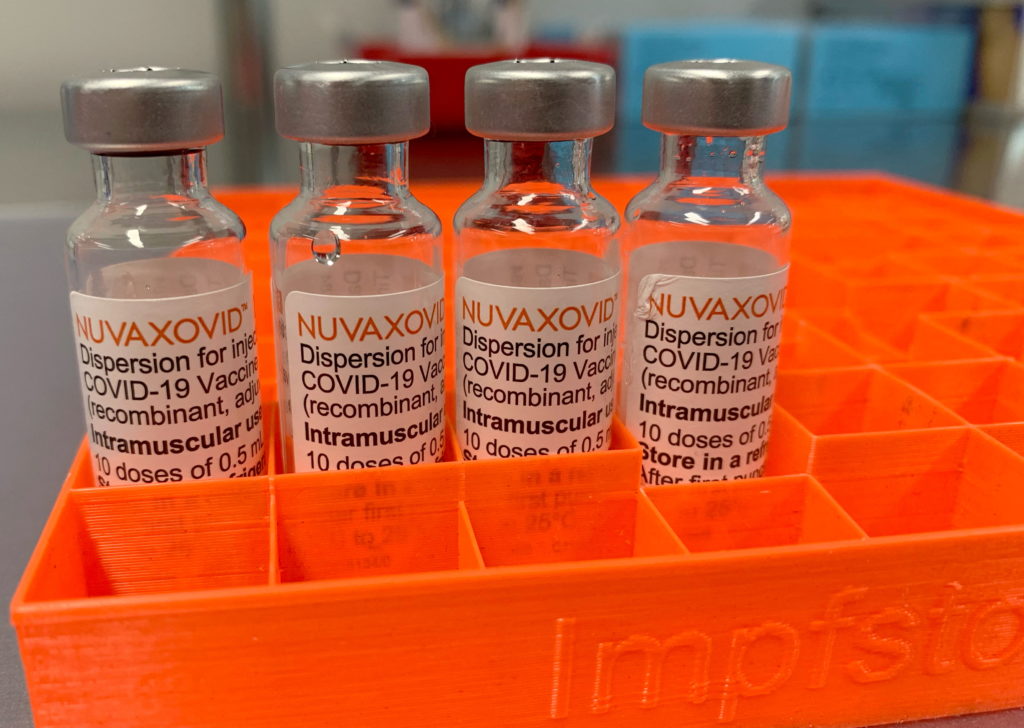
The FDA has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, offering a new option for individuals seeking protection against the virus. This protein-subunit vaccine, distinct from mRNA vaccines like Pfizer-BioNTech and Moderna, has been eagerly anticipated, particularly by those hesitant about the newer technologies. However, understanding the nuances of its usage restrictions is crucial before scheduling your vaccination.
What Makes Novavax Different?
Unlike mRNA vaccines, which use messenger RNA to instruct cells to produce a viral protein, Novavax utilizes a more traditional approach. Nuvaxovid contains a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein is combined with an adjuvant, a substance that boosts the immune response. This method has been used in other vaccines for decades, potentially appealing to individuals concerned about the novelty of mRNA technology. Learn more about the differences between vaccine types on the .
FDA Approval and Usage Restrictions:
The FDA's EUA is not a full approval, but it does signify that the vaccine meets rigorous safety and effectiveness standards for emergency use. However, certain usage restrictions are in place:
- Age: Currently, the vaccine is authorized for individuals 18 years of age and older. Further trials are underway to assess its safety and efficacy in younger age groups.
- Dosage: The vaccine is administered as a two-dose primary series, given three to eight weeks apart.
- Pre-existing Conditions: Individuals with known allergies to any component of the vaccine should not receive it. As with other COVID-19 vaccines, those with a history of severe allergic reactions should consult their physician before vaccination.
- Pregnancy and Breastfeeding: While data on pregnant and breastfeeding individuals is limited, the FDA acknowledges the potential benefits of vaccination in this population and encourages consultation with healthcare providers. .
Potential Side Effects:
Like other COVID-19 vaccines, Nuvaxovid can cause side effects, though most are mild and temporary. Common side effects include pain, redness, or swelling at the injection site, fatigue, headache, muscle aches, and nausea. Severe side effects are rare.
The Importance of Vaccination:
The FDA's approval of the Novavax vaccine provides another critical tool in the fight against COVID-19. Vaccination remains one of the most effective strategies for protecting individuals from severe illness, hospitalization, and death due to COVID-19.
Where to Get Vaccinated:
You can find vaccination sites near you by visiting the . Talk to your doctor or healthcare provider to discuss whether the Novavax vaccine is the right choice for you. Remember, making an informed decision about vaccination is crucial for protecting yourself and your community.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine EUA, protein-subunit vaccine, mRNA vaccine, vaccination, side effects, COVID-19, usage restrictions, vaccine safety, vaccine efficacy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Usage Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Luxury Gift Uzi League Of Legends Player Awarded Electric G Wagon By Mercedes Benz
May 21, 2025
Luxury Gift Uzi League Of Legends Player Awarded Electric G Wagon By Mercedes Benz
May 21, 2025 -
 Juvenile Arrests Follow Church Break In Bathroom Defilement
May 21, 2025
Juvenile Arrests Follow Church Break In Bathroom Defilement
May 21, 2025 -
 Intense Rainfall And Severe Storms Forecast For North Carolina Tonight
May 21, 2025
Intense Rainfall And Severe Storms Forecast For North Carolina Tonight
May 21, 2025 -
 North Carolina Under Severe Weather Risk Heavy Rain And Storms Expected Overnight
May 21, 2025
North Carolina Under Severe Weather Risk Heavy Rain And Storms Expected Overnight
May 21, 2025 -
 Cease Fire Efforts Trump To Speak With Putin And Zelensky As Russia Steps Up Attacks On Ukraine
May 21, 2025
Cease Fire Efforts Trump To Speak With Putin And Zelensky As Russia Steps Up Attacks On Ukraine
May 21, 2025
