FDA Approval For Novavax COVID-19 Vaccine: Understanding The Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Understanding the Usage Restrictions
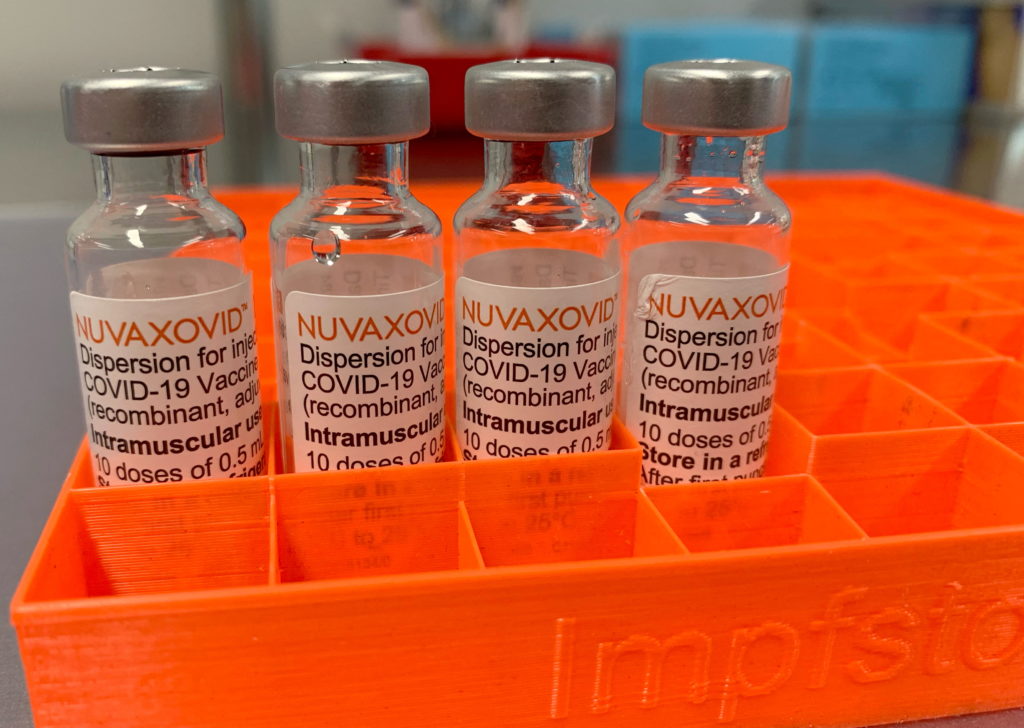
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marked a significant milestone in the fight against the pandemic. However, understanding the nuances of its usage restrictions is crucial for both healthcare providers and the public. This article delves into the specifics of the approval, highlighting key limitations and providing clarity on who can and cannot receive this particular vaccine.
A Closer Look at the Novavax Approval:
The Novavax vaccine utilizes a different technology compared to the mRNA vaccines from Pfizer-BioNTech and Moderna. It employs a protein subunit approach, using lab-grown pieces of the virus to trigger an immune response. This protein-based technology has been used in other vaccines for years, potentially appealing to individuals hesitant about mRNA vaccines. The FDA's approval, while welcome, comes with specific limitations.
Key Usage Restrictions:
-
Age Restrictions: Currently, the Novavax vaccine is authorized for individuals 18 years of age and older. Unlike some other COVID-19 vaccines, it is not yet approved for use in adolescents or children. Further trials are underway to determine its safety and efficacy in younger populations.
-
Booster Shots: While initially approved for the primary vaccination series (two doses), the FDA's authorization doesn't yet extend to booster shots. Further research is needed to assess the long-term efficacy and safety of Novavax as a booster. Stay updated on announcements from the CDC and FDA regarding booster authorization.
-
Pre-existing Conditions: As with any vaccine, individuals with specific pre-existing conditions should consult their physician before receiving the Novavax vaccine. While generally safe, certain allergies or medical history could influence the decision. Always disclose any relevant medical information to your healthcare provider.
-
Allergic Reactions: While rare, allergic reactions to the Novavax vaccine are possible. Individuals with a history of severe allergic reactions to any vaccine component should exercise caution and discuss their concerns with a doctor. Medical facilities administering the vaccine are equipped to handle such reactions.
-
Efficacy Against Variants: The efficacy of the Novavax vaccine against emerging COVID-19 variants is continuously monitored. While effective against the original strain, its performance against newer variants might differ. The FDA and CDC will provide updates on its efficacy as new data becomes available. Staying informed about variant prevalence in your area is important.
Comparing Novavax to Other COVID-19 Vaccines:
The availability of Novavax offers an alternative for individuals who prefer a protein-based vaccine. It's important to remember that all authorized COVID-19 vaccines have demonstrated significant efficacy in preventing severe illness, hospitalization, and death. The choice of vaccine should be made in consultation with a healthcare provider, considering individual medical history and preferences. Learn more about the different COVID-19 vaccines available on the .
Staying Informed and Making Informed Decisions:
The FDA and CDC continue to monitor the safety and efficacy of all authorized COVID-19 vaccines, including Novavax. Regularly checking their websites for updates and guidance is essential. Remember to discuss any concerns or questions with your healthcare provider to make an informed decision about COVID-19 vaccination. Your health and wellbeing are paramount.
Call to Action: Consult your doctor to determine if the Novavax vaccine is the right choice for you, considering your individual health circumstances and risk factors. Staying informed and proactive is crucial in navigating the evolving COVID-19 landscape.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Understanding The Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Walmart Faces Trumps Tariff Challenge Higher Prices Or Absorption
May 20, 2025
Walmart Faces Trumps Tariff Challenge Higher Prices Or Absorption
May 20, 2025 -
 Major Solar Storm Triggers Continent Wide Communications Breakdown
May 20, 2025
Major Solar Storm Triggers Continent Wide Communications Breakdown
May 20, 2025 -
 A J Perez On The Pressure And Threats Surrounding The Brett Favre Untold Documentary
May 20, 2025
A J Perez On The Pressure And Threats Surrounding The Brett Favre Untold Documentary
May 20, 2025 -
 Us Treasury Yield Decline Follows Feds Rate Cut Prediction
May 20, 2025
Us Treasury Yield Decline Follows Feds Rate Cut Prediction
May 20, 2025 -
 The Putin Trump Relationship A Power Imbalance Revealed
May 20, 2025
The Putin Trump Relationship A Power Imbalance Revealed
May 20, 2025
