FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Understanding the Restrictions
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marked a significant milestone in the fight against the pandemic. However, this approval isn't without its limitations. Understanding these restrictions is crucial for both healthcare professionals and the public to make informed decisions about vaccination. This article will delve into the specifics of the FDA's authorization, highlighting the key restrictions and their implications.
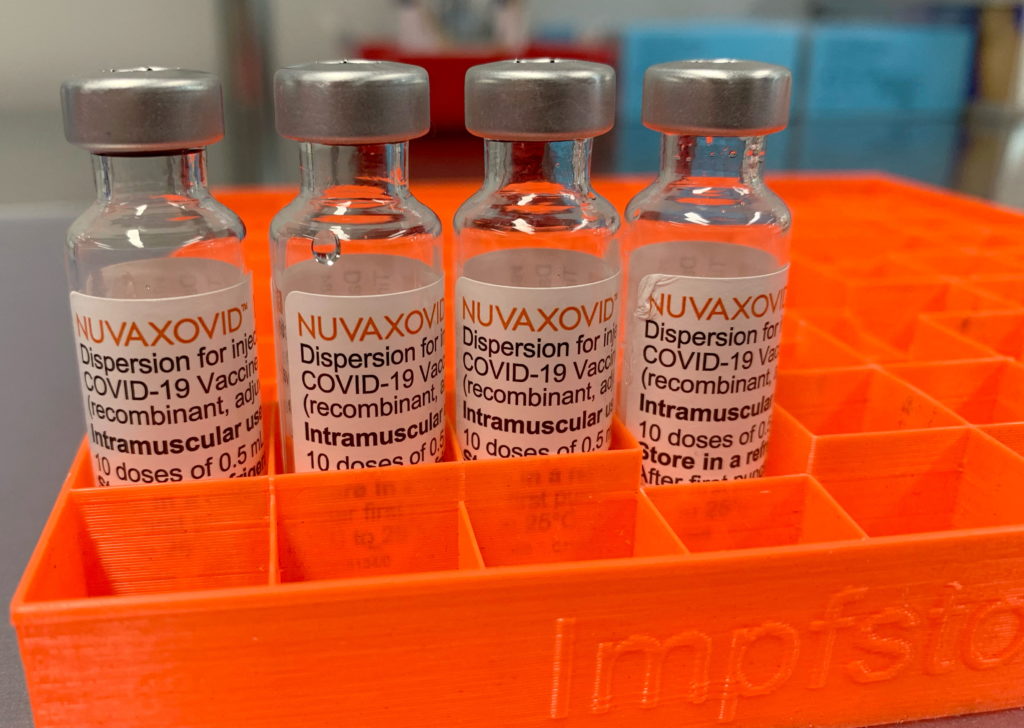
What is Nuvaxovid?
Nuvaxovid, developed by Novavax, is a protein subunit COVID-19 vaccine. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, it uses a different technology. It employs a lab-made version of the coronavirus spike protein to trigger an immune response. This protein-based approach has been touted by some as a potentially more palatable option for individuals hesitant about mRNA technology. This difference in technology is a key aspect to consider when examining the FDA's approval and subsequent restrictions.
FDA Approval: A Closer Look
The FDA granted Emergency Use Authorization (EUA) to Nuvaxovid earlier in the pandemic, but the recent approval represents a significant step forward, indicating a higher level of review and scrutiny. However, the approval wasn't unconditional. The restrictions imposed reflect ongoing data collection and the need for continued monitoring of the vaccine's safety and efficacy.
Key Restrictions and Their Implications:
-
Age Limitations: Initially, the FDA approval was limited to certain age groups. While the specific age ranges may vary, it's crucial to check the latest FDA guidelines for the most up-to-date information. This age restriction reflects the data available at the time of approval; further studies may expand the eligible age range in the future.
-
Dosage and Administration: Specific dosage recommendations and administration protocols were outlined by the FDA. Healthcare providers must strictly adhere to these guidelines to ensure the vaccine's safety and efficacy. Deviation from these protocols could compromise the vaccine's effectiveness and potentially lead to adverse events.
-
Monitoring for Adverse Events: Post-market surveillance remains a critical component of the FDA's ongoing assessment of Nuvaxovid. Healthcare professionals are responsible for reporting any suspected adverse events to the Vaccine Adverse Event Reporting System (VAERS). This continuous monitoring is essential for identifying and addressing any potential safety concerns.
-
Specific Populations: The FDA may impose additional restrictions for specific populations, such as individuals with certain pre-existing conditions or those undergoing specific treatments. Consult your physician to determine if Nuvaxovid is appropriate for your individual circumstances.
Comparing Nuvaxovid to Other COVID-19 Vaccines:
While Nuvaxovid offers an alternative technology to mRNA vaccines, it's essential to remember that all authorized COVID-19 vaccines have proven effective in preventing severe illness, hospitalization, and death. The choice between different vaccines should be based on individual preferences, health status, and consultation with a healthcare provider. More information on comparing vaccine types can be found on the .
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine is a welcome development in the global fight against COVID-19. However, understanding the associated restrictions is vital for making informed decisions. Always consult with your healthcare provider to determine which COVID-19 vaccine is best suited for your individual needs and circumstances. Staying informed about the latest FDA guidelines and updates is crucial in navigating this evolving landscape. Remember to check the for the most current information.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Understanding The Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Derrys Nightmare Returns It Chapter Two Trailer Released
May 20, 2025
Derrys Nightmare Returns It Chapter Two Trailer Released
May 20, 2025 -
 5 Billion Poured Into Bitcoin Etfs What This Means For The Future
May 20, 2025
5 Billion Poured Into Bitcoin Etfs What This Means For The Future
May 20, 2025 -
 Ukraine War Trump Pushes For Immediate Peace Negotiations With Russia
May 20, 2025
Ukraine War Trump Pushes For Immediate Peace Negotiations With Russia
May 20, 2025 -
 Marsh Markram Power Srh To 206 Lucknows Must Win Game
May 20, 2025
Marsh Markram Power Srh To 206 Lucknows Must Win Game
May 20, 2025 -
 Star Wars Battlefront A Surprise Return To The Gaming Scene
May 20, 2025
Star Wars Battlefront A Surprise Return To The Gaming Scene
May 20, 2025
