FDA Approval For Novavax COVID-19 Vaccine: Understanding The Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Understanding the Conditions
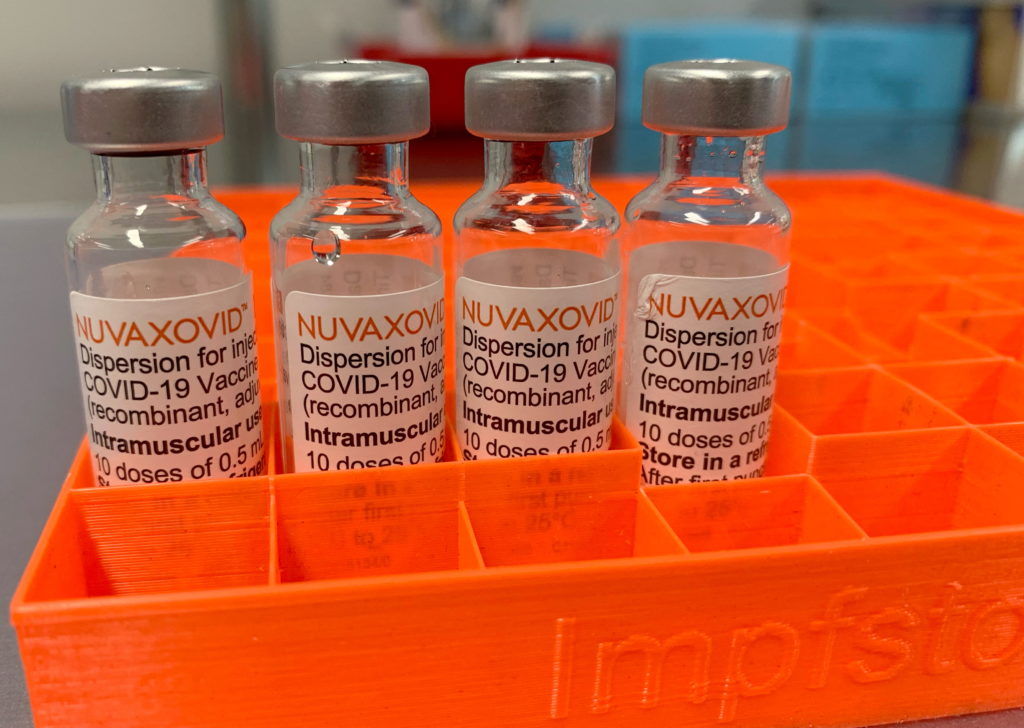
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant milestone in the fight against the pandemic. However, it's crucial to understand the conditions surrounding this approval to accurately assess its role in the ongoing vaccination efforts. This isn't just another vaccine; its unique protein-based technology differentiates it from the mRNA vaccines already widely available, potentially making it a game-changer for vaccine hesitant individuals.
What Makes Novavax Different?
Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Novavax uses a more traditional approach. Nuvaxovid is a protein subunit vaccine. This means it uses harmless pieces of the virus (spike proteins) to trigger an immune response. This technology has been used for decades in vaccines against other diseases like Hepatitis B and HPV, potentially fostering greater public trust due to its familiarity. This difference is key for those who have expressed concerns about the novel mRNA technology.
The FDA Approval: A Closer Look
The FDA's approval wasn't unconditional. The authorization is specifically for individuals 18 years of age and older. Further studies are needed to determine its efficacy and safety in younger age groups. This phased approach is standard practice for new vaccines, ensuring thorough safety data is collected before broader rollout. The approval also comes with rigorous manufacturing and quality control oversight by the FDA to maintain consistent product quality and efficacy.
Addressing Concerns and Misconceptions
Several misconceptions surround the Novavax vaccine. One common concern is its perceived "late arrival" to the market. While it arrived later than mRNA vaccines, this delay was due to rigorous testing and development processes, aiming for optimal safety and efficacy. The delay doesn't automatically equate to inferiority; rather, it highlights a commitment to rigorous scientific standards.
Another point to consider is the vaccine's efficacy. While clinical trials showed high efficacy against COVID-19, the real-world effectiveness might vary depending on the circulating variants. The FDA, alongside the CDC, will continue to monitor its performance and adjust recommendations as needed. [Link to CDC website on vaccine effectiveness].
The Role of Novavax in the Vaccination Strategy
The addition of Novavax to the arsenal of available COVID-19 vaccines broadens the options for individuals and healthcare providers. Its protein subunit technology offers a compelling alternative for those who have been hesitant to receive mRNA vaccines. This could potentially increase vaccination rates and contribute significantly to achieving broader population immunity. However, it’s essential to remember that no vaccine is 100% effective, and adherence to public health guidelines such as mask-wearing and social distancing remains important.
What's Next?
Further research will focus on extending approval to younger age groups and evaluating long-term effectiveness and safety. Ongoing monitoring by regulatory agencies will ensure the vaccine remains safe and effective against emerging variants. The availability of Novavax represents a crucial step forward in the global fight against COVID-19, offering a valuable alternative to existing vaccine technologies. The FDA's approval, while conditional, signifies a positive development in our efforts to control the pandemic.
Keywords: Novavax, COVID-19 vaccine, FDA approval, protein subunit vaccine, mRNA vaccine, vaccine efficacy, vaccine safety, Nuvaxovid, vaccination, public health, pandemic, COVID-19 variants.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Understanding The Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Bitcoin Etf Investments Surge Past 5 Billion Analyzing The Market Trend
May 20, 2025
Bitcoin Etf Investments Surge Past 5 Billion Analyzing The Market Trend
May 20, 2025 -
 Peaky Blinders Creator Announces New Series With Significant Shift
May 20, 2025
Peaky Blinders Creator Announces New Series With Significant Shift
May 20, 2025 -
 Nick Sirianni Gets A Well Deserved Extension Analyzing The Eagles Head Coach Deal
May 20, 2025
Nick Sirianni Gets A Well Deserved Extension Analyzing The Eagles Head Coach Deal
May 20, 2025 -
 Australias Interest Rate Cut A Response To Easing Inflation
May 20, 2025
Australias Interest Rate Cut A Response To Easing Inflation
May 20, 2025 -
 I M Done Jon Jones Hints At Retirement Amidst Aspinall Fight Delays
May 20, 2025
I M Done Jon Jones Hints At Retirement Amidst Aspinall Fight Delays
May 20, 2025
