FDA Approval For Novavax COVID-19 Vaccine: Stricter Guidelines In Place
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
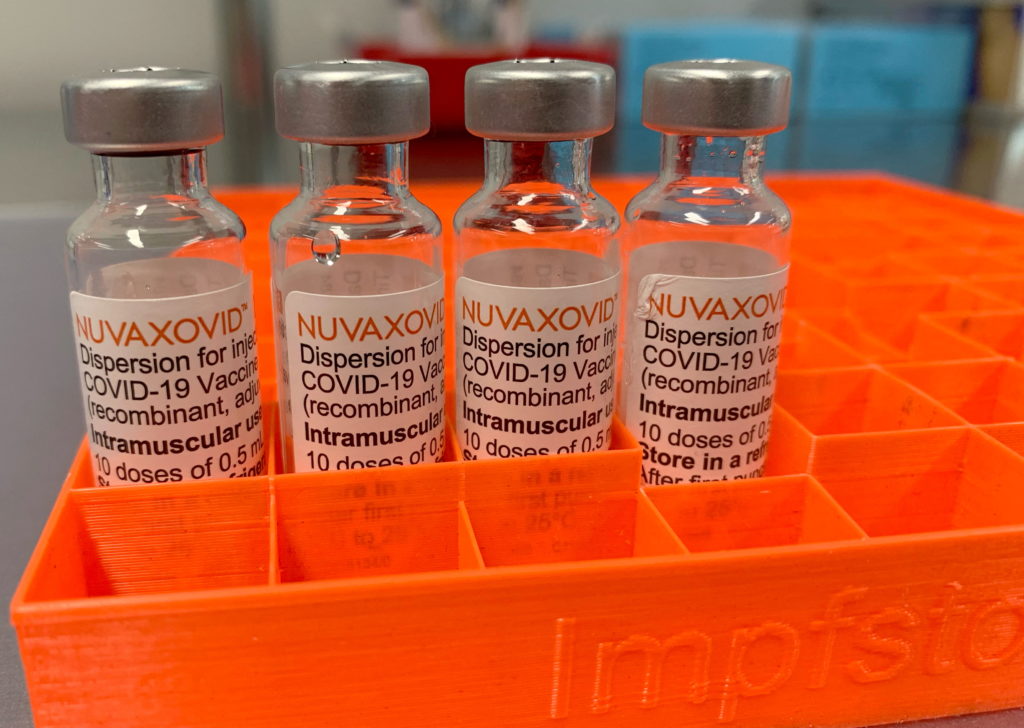
FDA Approval for Novavax COVID-19 Vaccine: Stricter Guidelines in Place
The FDA's approval of the Novavax COVID-19 vaccine marks a significant step in the fight against the pandemic, but with this approval come stricter guidelines than initially anticipated. This development offers a new option for those hesitant about mRNA vaccines, but understanding the nuances of the approval and its accompanying regulations is crucial.
A Different Approach to Vaccine Technology
Unlike the Pfizer-BioNTech and Moderna vaccines, which utilize mRNA technology, Novavax's Nuvaxovid employs a more traditional protein subunit approach. This method uses purified proteins from the virus to trigger an immune response, potentially addressing concerns some individuals had about mRNA vaccines. This difference in technology makes it a valuable addition to the arsenal of COVID-19 vaccines, offering an alternative for those seeking a non-mRNA option.
Stricter FDA Guidelines: What They Mean for Patients
While the approval is welcome news, the FDA has implemented stricter guidelines for Novavax's distribution and use. These guidelines reflect a cautious approach, prioritizing patient safety and efficacy. The specifics of these guidelines are still being disseminated, but key elements likely include:
- Enhanced Monitoring: Expect more rigorous monitoring of adverse events following vaccination. This includes increased reporting requirements for healthcare providers and a more robust data collection system to track potential side effects.
- Targeted Distribution: Initial distribution might be targeted towards specific demographics or regions, depending on demand and logistical considerations. This strategy aims to optimize vaccine allocation and ensure effective deployment.
- Clearer Communication: The FDA will likely emphasize clearer communication regarding the vaccine's benefits and risks to patients and healthcare professionals. This includes providing easily accessible information about potential side effects and contraindications.
Addressing Vaccine Hesitancy and Boosting Vaccination Rates
The FDA's approval, despite the stricter guidelines, aims to address vaccine hesitancy and contribute to higher vaccination rates. Many individuals have expressed concerns about the speed of mRNA vaccine development and deployment. The more traditional approach of the Novavax vaccine may alleviate these concerns for some, encouraging them to get vaccinated. However, it's crucial to remember that all COVID-19 vaccines currently authorized have undergone rigorous testing and have proven effective in preventing severe illness, hospitalization, and death.
Looking Ahead: The Role of Novavax in the Ongoing Pandemic
The arrival of the Novavax vaccine, with its distinct technology and accompanying guidelines, presents a significant development in the ongoing pandemic. Its impact on global vaccination efforts and the fight against COVID-19 variants remains to be seen. Continued monitoring of its efficacy and safety will be essential, and the FDA’s stringent guidelines suggest a commitment to ensuring its safe and effective deployment.
Further Resources:
Call to Action: Consult your healthcare provider to determine if the Novavax COVID-19 vaccine is the right choice for you. Staying informed about the latest vaccine information is crucial in protecting yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Stricter Guidelines In Place. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Remembering Latonya Pottain My 600 Lb Life Star Passes Away
May 20, 2025
Remembering Latonya Pottain My 600 Lb Life Star Passes Away
May 20, 2025 -
 Beyond Freaky Friday Jamie Lee Curtis Shares Insights Into Her Friendship With Lindsay Lohan
May 20, 2025
Beyond Freaky Friday Jamie Lee Curtis Shares Insights Into Her Friendship With Lindsay Lohan
May 20, 2025 -
 World Leaders Send Messages Of Support To President Biden Amidst Cancer Battle
May 20, 2025
World Leaders Send Messages Of Support To President Biden Amidst Cancer Battle
May 20, 2025 -
 Putins Actions Highlight Trumps Reduced Global Relevance
May 20, 2025
Putins Actions Highlight Trumps Reduced Global Relevance
May 20, 2025 -
 The Impact Of Climate Change On Pregnancy Challenges And Adaptions For A Changing World
May 20, 2025
The Impact Of Climate Change On Pregnancy Challenges And Adaptions For A Changing World
May 20, 2025
