FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
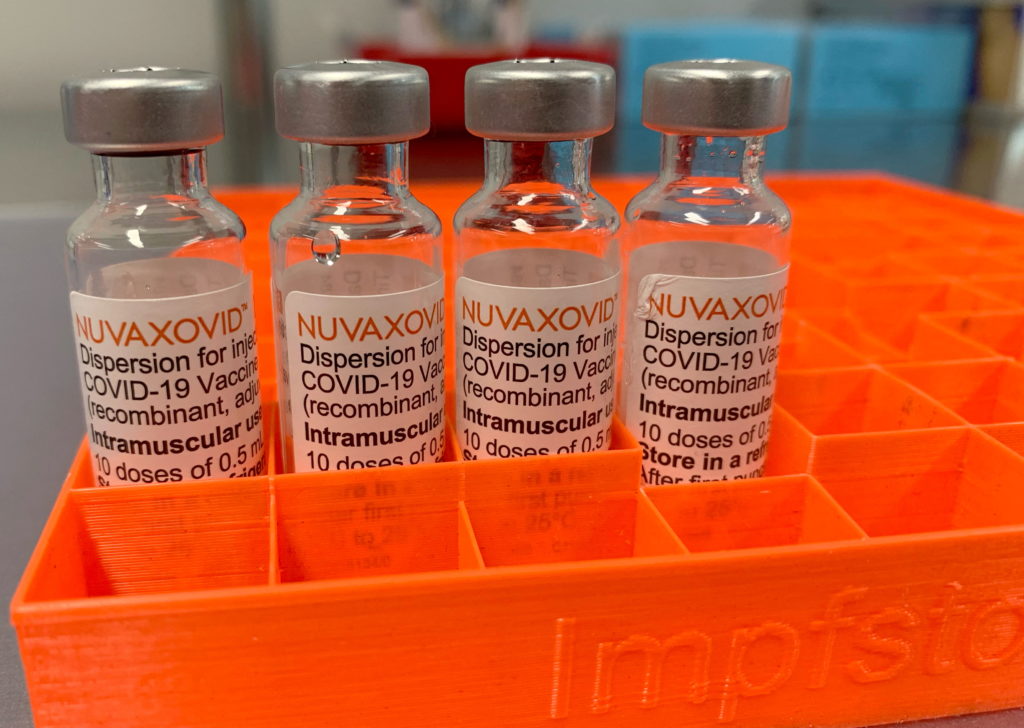
FDA Approval for Novavax COVID-19 Vaccine Comes With Unusual Limitations
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, was met with a mixture of relief and confusion. While advocates celebrated the addition of a new vaccine option, the approval came with significant limitations, raising questions about its practical use and market impact. This unprecedented approach warrants a closer look.
A New Contender, But With Caveats:
Novavax's protein-subunit vaccine technology differs from the mRNA vaccines developed by Pfizer-BioNTech and Moderna. This difference, while initially touted as a potential advantage for those hesitant about mRNA technology, didn't translate into a straightforward approval process. The FDA's authorization is significantly narrower than those granted to its competitors.
Unusual Limitations Explained:
The FDA's approval is limited to individuals 18 years of age and older. This contrasts sharply with the wider age ranges covered by the Pfizer and Moderna vaccines. Furthermore, the agency imposed limitations on the vaccine's authorized uses, restricting it to individuals who haven't already received another COVID-19 vaccine. This essentially carves out a small niche for Nuvaxovid within the existing vaccination landscape.
Why the Restrictions?
The FDA cited several reasons for these unusual limitations. While the vaccine demonstrated efficacy in clinical trials, the data supporting its use in younger populations or as a booster dose is currently lacking. The agency emphasized the need for further studies to fully assess Nuvaxovid's safety and effectiveness across a broader range of demographics and usage scenarios. This cautious approach reflects the FDA's commitment to rigorous scientific evaluation, but also highlights the challenges faced by late-to-market vaccine candidates in a landscape already dominated by established options.
Impact on Vaccination Strategy:
The limited approval raises concerns about the vaccine's overall impact on the public health response to COVID-19. With many individuals already vaccinated with other options, the pool of potential recipients for Nuvaxovid is considerably smaller. This may limit its contribution to overall vaccination rates and herd immunity.
Future Prospects:
Novavax has expressed its intention to conduct further clinical trials to address the FDA's concerns and potentially expand the vaccine's authorized use. The success of these trials will be crucial in determining the long-term role of Nuvaxovid in the global COVID-19 vaccination strategy. The company might also explore different formulations or strategies to broaden its applicability and compete more effectively in the already saturated market.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine limitations, protein-subunit vaccine, mRNA vaccine, vaccination strategy, public health, clinical trials.
Call to Action: Stay informed about the latest developments in COVID-19 vaccination by regularly checking the CDC and FDA websites for updates. Consult your doctor to discuss which COVID-19 vaccine is most appropriate for your individual needs.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Bali Tightens Tourist Regulations To Preserve Cultural Heritage And Natural Beauty
May 20, 2025
Bali Tightens Tourist Regulations To Preserve Cultural Heritage And Natural Beauty
May 20, 2025 -
 Bali Tourism Overhaul New Guidelines Aim To Improve Tourist Conduct
May 20, 2025
Bali Tourism Overhaul New Guidelines Aim To Improve Tourist Conduct
May 20, 2025 -
 A Deep Dive Into American Medical And Scientific Research Progress Challenges And Opportunities
May 20, 2025
A Deep Dive Into American Medical And Scientific Research Progress Challenges And Opportunities
May 20, 2025 -
 Balis New Code Of Conduct Curbing Irresponsible Tourism
May 20, 2025
Balis New Code Of Conduct Curbing Irresponsible Tourism
May 20, 2025 -
 Venezuelan Migrants Lose Protected Status Supreme Court Backs Trump Era Decision
May 20, 2025
Venezuelan Migrants Lose Protected Status Supreme Court Backs Trump Era Decision
May 20, 2025
