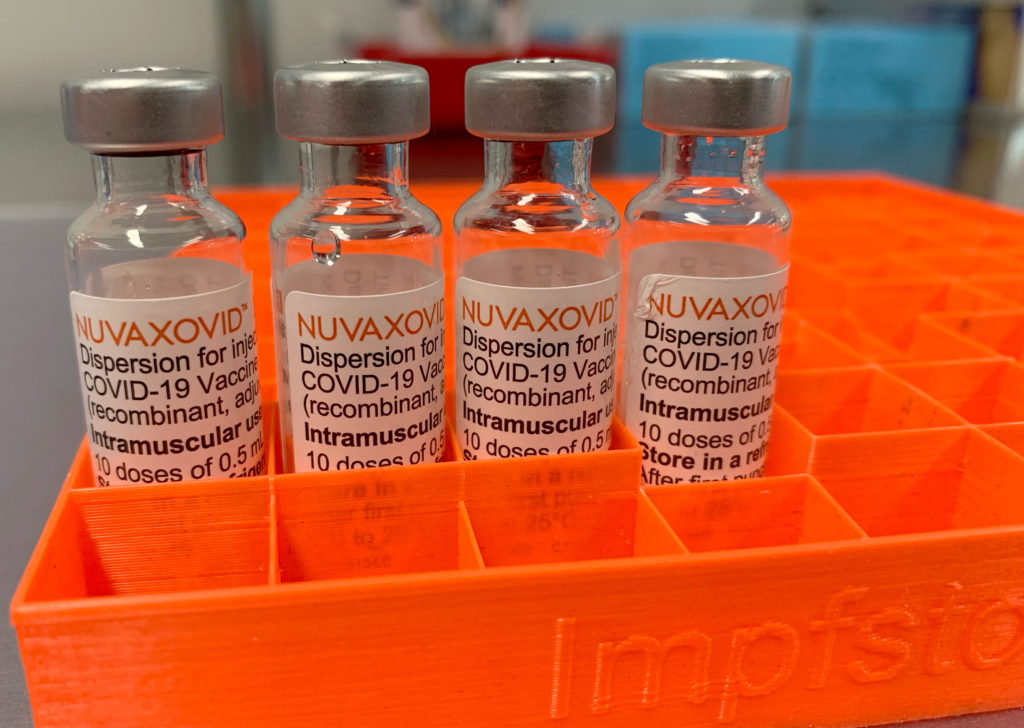
FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine Comes with Unusual Conditions
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mix of relief and cautious optimism. While the approval marks a significant step forward in the fight against the pandemic, the unusual conditions attached to it raise several questions. This landmark decision, announced [insert date of announcement], comes with stipulations that differ significantly from previous COVID-19 vaccine authorizations.
Unprecedented Conditions for a Traditional Vaccine
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, Novavax's vaccine utilizes a more traditional protein subunit technology. This approach, considered by some to be safer due to its established history, has led many to anticipate a smoother regulatory process. However, the FDA's approval is contingent upon several unusual conditions, prompting scrutiny from experts and the public alike.
One notable condition involves the ongoing monitoring of myocarditis and pericarditis, inflammation of the heart muscle and lining, respectively. While these conditions have been linked to other COVID-19 vaccines, particularly in younger males, the FDA is implementing heightened surveillance for Novavax's vaccine as a precautionary measure. This ongoing monitoring will involve a rigorous data collection and analysis process, adding a layer of complexity to the vaccine's rollout.
Furthermore, the FDA has imposed strict manufacturing requirements, emphasizing the need for consistent quality control throughout the production process. This underscores the agency's commitment to ensuring the safety and efficacy of the vaccine, but also highlights potential challenges in scaling up production to meet anticipated demand.
What does this mean for vaccine uptake?
The unusual conditions attached to the Novavax vaccine's approval may impact public perception and vaccine uptake. Concerns about potential side effects, however rare, can influence vaccine hesitancy. The FDA's transparent approach to ongoing monitoring is a positive step in addressing these concerns. However, effective communication strategies are crucial to build public trust and ensure the vaccine reaches those who need it most. Public health officials and healthcare providers will need to clearly and concisely convey the benefits and risks, emphasizing that the FDA's approval, despite the conditions, signifies a rigorous evaluation process.
Looking Ahead: The Future of Novavax and COVID-19 Vaccination
The FDA's approval of the Novavax vaccine, albeit with unusual conditions, is a significant development in the ongoing fight against COVID-19. The protein subunit technology offers a different approach to vaccination, potentially catering to individuals hesitant about mRNA vaccines. The stringent monitoring and manufacturing requirements, while demanding, underscore the FDA's commitment to vaccine safety and efficacy.
The success of the Novavax vaccine will largely depend on addressing public concerns, ensuring efficient distribution, and maintaining transparent communication about any emerging safety data. The future of COVID-19 vaccination strategies may well hinge on the performance and acceptance of this new vaccine option. Further research and analysis will be crucial in understanding the long-term effects and efficacy of Nuvaxovid in various populations. Stay informed by consulting reputable sources like the [link to CDC website] and [link to FDA website] for updated information and guidance on COVID-19 vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Unusual Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 One Rate Cut Predicted For 2025 Impact On U S Treasury Yields
May 20, 2025
One Rate Cut Predicted For 2025 Impact On U S Treasury Yields
May 20, 2025 -
 Valley Outdoorsmen Dispute Georgia Womans Fresno County Survival Account
May 20, 2025
Valley Outdoorsmen Dispute Georgia Womans Fresno County Survival Account
May 20, 2025 -
 Srh Post 206 Run Target Against Lsg Marsh And Markrams Crucial Fifties
May 20, 2025
Srh Post 206 Run Target Against Lsg Marsh And Markrams Crucial Fifties
May 20, 2025 -
 Investor Confidence In Ethereum Boosted 200 M Investment After Pectra
May 20, 2025
Investor Confidence In Ethereum Boosted 200 M Investment After Pectra
May 20, 2025 -
 Balis Tourism Safety A Call For International Assistance
May 20, 2025
Balis Tourism Safety A Call For International Assistance
May 20, 2025
