FDA Approval For Novavax COVID-19 Vaccine Comes With Strict Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
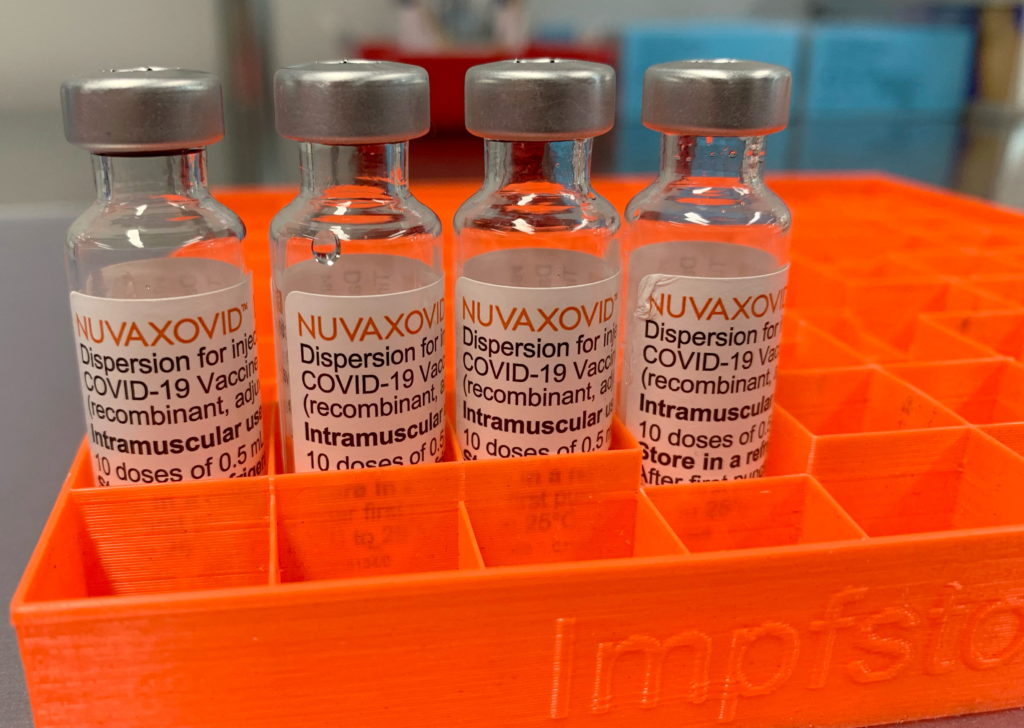
FDA Approval for Novavax COVID-19 Vaccine Comes with Strict Conditions
The long-awaited FDA approval of the Novavax COVID-19 vaccine has finally arrived, but with significant caveats. After months of delay and scrutiny, the US Food and Drug Administration (FDA) has granted full approval to the protein-subunit vaccine, Nuvaxovid. However, this approval comes with strict conditions, raising questions about its immediate impact and future role in the ongoing pandemic response.
The FDA's decision marks a significant milestone for Novavax, offering a different approach to COVID-19 vaccination compared to the mRNA vaccines from Pfizer-BioNTech and Moderna. Nuvaxovid uses a more traditional technology, employing a protein subunit of the virus to trigger an immune response. This approach has been seen by some as potentially appealing to vaccine-hesitant individuals. However, the stringent conditions attached to the approval highlight concerns regarding the vaccine's efficacy and safety profile compared to its competitors.
What are the Conditions of Approval?
The FDA's approval isn't unconditional. Key conditions include:
- Post-Market Surveillance: Novavax is required to conduct extensive post-market surveillance to continuously monitor the vaccine's safety and effectiveness. This will involve tracking adverse events and comparing its performance to other authorized COVID-19 vaccines.
- Manufacturing Requirements: The FDA has imposed strict requirements on the manufacturing process to ensure consistent quality and safety. Any deviations from these standards could lead to further regulatory action.
- Ongoing Data Collection: Novavax must continue to collect and submit data on the vaccine's performance, including real-world effectiveness data from various populations. This ongoing data collection is crucial for assessing long-term efficacy and safety.
Why the Strict Conditions?
The FDA's stringent approach is likely due to several factors:
- Delayed Clinical Trials: The Novavax vaccine's clinical trial data was submitted later than the mRNA vaccines, leading to a more thorough review process.
- Lower Efficacy Rates: Some studies have shown lower efficacy rates for the Novavax vaccine compared to mRNA vaccines, particularly against certain variants.
- Concerns about Rare Side Effects: While generally well-tolerated, concerns about rare side effects may have contributed to the need for rigorous post-market surveillance.
What Does This Mean for the Public?
The approval of Nuvaxovid offers another option for those seeking COVID-19 vaccination. However, its limited availability and the FDA's strict conditions mean it might not immediately become a widespread choice. Individuals should consult with their healthcare providers to determine the most suitable vaccine for their individual needs and risk factors. The ongoing monitoring will be crucial in assessing the long-term benefits and risks of the Novavax vaccine.
Looking Ahead: The Future of Novavax and COVID-19 Vaccination
The FDA's approval, while significant, doesn't guarantee the widespread adoption of the Novavax vaccine. Its efficacy compared to existing vaccines, alongside the strict regulatory oversight, will ultimately determine its role in the ongoing fight against COVID-19. The coming months will be critical in observing the post-market data and assessing the long-term impact of this newly approved vaccine. Further research and updates on the vaccine's performance are eagerly awaited. For up-to-date information on COVID-19 vaccines and prevention strategies, visit the .
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-subunit vaccine, vaccine safety, vaccine efficacy, post-market surveillance, COVID-19 vaccination, mRNA vaccines, pandemic response, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine Comes With Strict Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Freaky Friday Reunion Jamie Lee Curtis Reveals Current Status With Lindsay Lohan
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis Reveals Current Status With Lindsay Lohan
May 20, 2025 -
 Jamie Lee Curtis Lindsay Lohan Has Always Kept It Real
May 20, 2025
Jamie Lee Curtis Lindsay Lohan Has Always Kept It Real
May 20, 2025 -
 Major Solar Flare Disrupts Radio In Europe Asia And The Middle East
May 20, 2025
Major Solar Flare Disrupts Radio In Europe Asia And The Middle East
May 20, 2025 -
 From Labs To Lives How Us Medical And Scientific Research Benefits The Nation
May 20, 2025
From Labs To Lives How Us Medical And Scientific Research Benefits The Nation
May 20, 2025 -
 65 000 Airbnb Rentals In Spain Face Closure Order
May 20, 2025
65 000 Airbnb Rentals In Spain Face Closure Order
May 20, 2025
