Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Understanding the Novavax COVID-19 Vaccine Restrictions
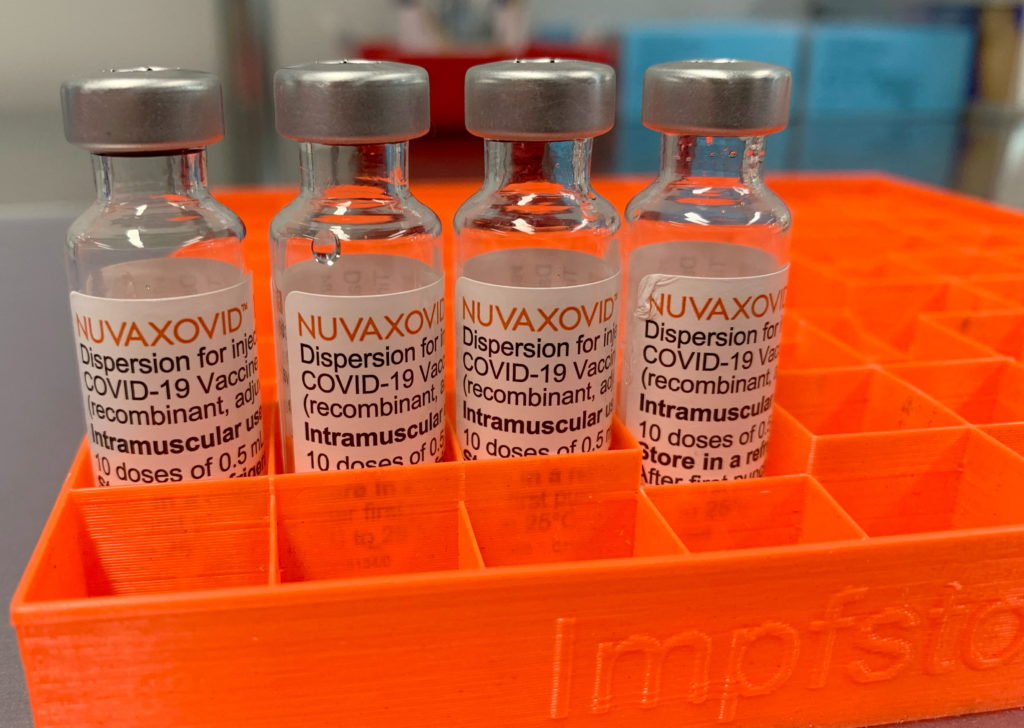
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, finally gained conditional FDA approval in July 2023, offering an alternative for individuals hesitant about mRNA vaccines. However, this approval wasn't without stipulations. Understanding these conditional approvals and the resulting restrictions is crucial for both healthcare providers and the public. This article delves into the specifics of the FDA's decision and what it means for vaccine distribution and usage.
What is Conditional FDA Approval?
Unlike a full FDA approval, which signifies a comprehensive review and confirmation of a drug's safety and efficacy, conditional approval allows for quicker access to potentially life-saving treatments during public health emergencies. This expedited pathway requires the manufacturer to continue providing data on the vaccine's long-term safety and efficacy post-market release. Essentially, the FDA grants approval conditioned upon the fulfillment of further requirements.
Novavax Vaccine: Restrictions and Conditions
The conditional approval of the Novavax COVID-19 vaccine came with specific restrictions and ongoing requirements for the manufacturer, Novavax, Inc. These include:
- Ongoing Safety Monitoring: Novavax is obligated to continuously monitor the vaccine's safety profile and submit regular reports to the FDA. This involves tracking adverse events and analyzing long-term data to assess potential risks.
- Further Clinical Trials: While initial trials demonstrated efficacy, the FDA may require additional studies to solidify the vaccine's safety and effectiveness across diverse populations. This continuous data gathering is a cornerstone of conditional approval.
- Manufacturing Standards: Strict manufacturing standards and quality control measures are mandatory to maintain the vaccine's consistency and potency. Any deviations must be immediately reported to the FDA.
- Labeling Requirements: The vaccine's labeling must accurately reflect its known benefits and risks, including any identified limitations or side effects. This transparency ensures informed consent from recipients.
Who Can Receive the Novavax Vaccine?
Currently, the Novavax vaccine is authorized for individuals 18 years of age and older. The FDA continues to evaluate its use in younger age groups. However, as with other COVID-19 vaccines, individuals with a history of severe allergic reactions to any vaccine component should consult with their healthcare provider before receiving the Novavax vaccine.
Why the Conditional Approval?
The conditional approval reflects the FDA's balance between the urgent need for diverse vaccine options during a pandemic and the necessity of thorough safety evaluation. The protein-based technology of the Novavax vaccine differs from the mRNA technology used in other leading COVID-19 vaccines, potentially appealing to individuals with concerns about mRNA vaccines. This alternative offers a valuable choice to increase vaccination rates and protect public health.
What Does this Mean for the Future?
The future of the Novavax vaccine hinges on Novavax's ability to meet the ongoing requirements imposed by the FDA. Continued safety monitoring and the submission of additional data will determine whether the conditional approval transitions to a full licensure. The availability and widespread adoption of the Novavax vaccine could significantly impact global vaccination efforts and contribute to the ongoing fight against COVID-19. Stay informed about updates from the FDA and your healthcare provider for the most up-to-date information.
Call to Action: Consult your doctor or healthcare provider to discuss the Novavax vaccine and determine if it's the right choice for you. Informed decisions are key to protecting your health and contributing to community immunity. For more information, visit the FDA website [link to FDA website].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Charlotte Residents Urged To Prepare For Overnight Storms And Temperature Plunge
May 21, 2025
Charlotte Residents Urged To Prepare For Overnight Storms And Temperature Plunge
May 21, 2025 -
 Reimagining The Bond How The Last Of Us Show Diverges From The Games Joel Ellie Relationship
May 21, 2025
Reimagining The Bond How The Last Of Us Show Diverges From The Games Joel Ellie Relationship
May 21, 2025 -
 Trumps Waning Power Putins Actions Highlight A Changing World Order
May 21, 2025
Trumps Waning Power Putins Actions Highlight A Changing World Order
May 21, 2025 -
 160 Japanese Firms Compete To Enhance Corporate Value Via Environmental Conservation
May 21, 2025
160 Japanese Firms Compete To Enhance Corporate Value Via Environmental Conservation
May 21, 2025 -
 Russia Ukraine Peace Talks Trumps Surprise Announcement
May 21, 2025
Russia Ukraine Peace Talks Trumps Surprise Announcement
May 21, 2025
