Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant development in the ongoing fight against the pandemic, but with caveats. This approval, while offering another option for vaccination, comes with restrictions that limit its immediate widespread use. Understanding these limitations is crucial for both healthcare professionals and the general public.
A New Contender in the COVID-19 Vaccine Landscape:
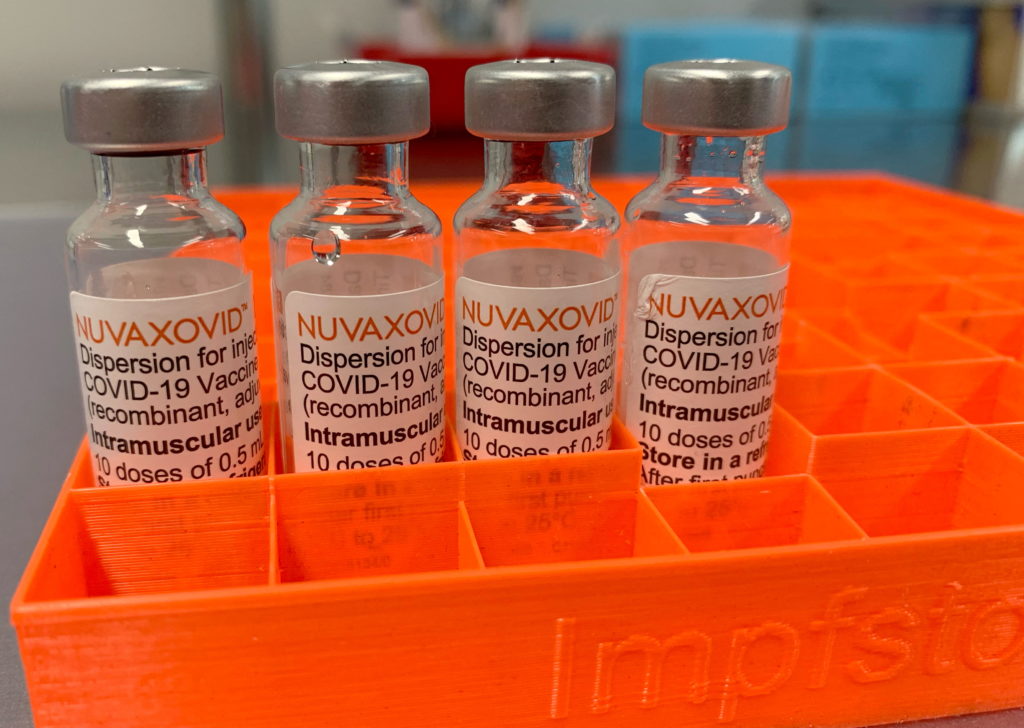
The Novavax vaccine, known as Nuvaxovid, uses a different technology than the mRNA vaccines (Pfizer-BioNTech and Moderna) and the viral vector vaccine (Johnson & Johnson). It utilizes a protein-based approach, employing a lab-grown version of the coronavirus spike protein to trigger an immune response. This protein-subunit technology has been used in other vaccines for decades, potentially appealing to individuals hesitant about mRNA technology. The FDA’s approval is based on extensive clinical trials demonstrating its efficacy and safety profile.
Understanding the Conditional Approval:
The FDA's approval is conditional, meaning it's granted based on ongoing monitoring of the vaccine's safety and effectiveness. This isn't an unusual approach for new vaccines, particularly during a public health emergency. The agency will continue to collect data on long-term effects and overall performance. This conditional approval also highlights a crucial point: the vaccine is not yet authorized for all age groups.
Restricted Use and Age Limits:
Currently, the Novavax vaccine is authorized for use in individuals 18 years of age and older. This age restriction stems from the limited data available from clinical trials involving younger populations. Further studies are needed before expanding the authorization to include adolescents and children. The FDA's decision emphasizes a cautious, data-driven approach, prioritizing safety across all demographics.
Why the Restricted Use? Key Considerations:
Several factors contribute to the conditional approval and restricted use:
- Limited Data on Long-Term Effects: While short-term efficacy and safety data are positive, longer-term monitoring is crucial to fully assess the vaccine's impact.
- Age-Specific Immune Responses: Immune responses to vaccines can vary across different age groups. More data is needed to ascertain the vaccine's effectiveness and safety in younger populations.
- Ongoing Pandemic Monitoring: The constantly evolving nature of the virus and the emergence of new variants necessitate ongoing vigilance and data collection for all COVID-19 vaccines.
What this Means for the Public:
The FDA's approval of the Novavax vaccine provides an additional tool in the fight against COVID-19. However, its current restricted use means it won't immediately be a widely available option for everyone. For adults 18 and older, it offers an alternative to existing vaccines. Individuals should consult with their healthcare providers to discuss which vaccine is most suitable for their individual circumstances and health profile.
Looking Ahead:
Further research and clinical trials are underway to expand the authorized use of the Novavax vaccine to include younger age groups. The FDA will continue to evaluate the data and adjust its authorization accordingly. The availability and accessibility of the Novavax vaccine will likely increase as these studies are completed and further data is gathered. For up-to-date information on COVID-19 vaccines, refer to the and .
Call to Action: Stay informed about COVID-19 vaccine developments and consult your healthcare provider for personalized vaccination advice.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Restricted Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Widespread Tornado Risk Severe Weather System Sweeping Across The Us
May 20, 2025
Widespread Tornado Risk Severe Weather System Sweeping Across The Us
May 20, 2025 -
 65 000 Airbnb Rentals In Spain Face Closure Order
May 20, 2025
65 000 Airbnb Rentals In Spain Face Closure Order
May 20, 2025 -
 Over 65 000 Airbnb Listings Suspended In Spain Due To Regulatory Non Compliance
May 20, 2025
Over 65 000 Airbnb Listings Suspended In Spain Due To Regulatory Non Compliance
May 20, 2025 -
 Brett Favres Legacy Under Scrutiny A Conversation With The Director Of Fall Of Favre
May 20, 2025
Brett Favres Legacy Under Scrutiny A Conversation With The Director Of Fall Of Favre
May 20, 2025 -
 Watch Now The Intense Ww 1 Drama Starring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
Watch Now The Intense Ww 1 Drama Starring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
