Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout
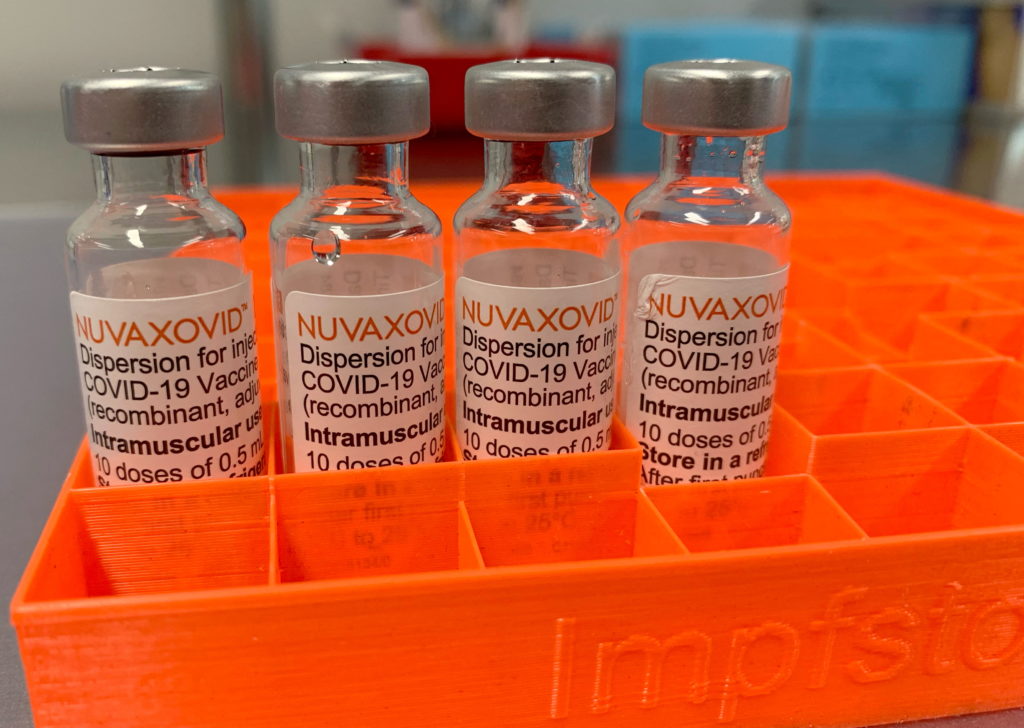
The FDA's conditional approval of the Novavax COVID-19 vaccine, NVX-CoV2373, has finally arrived, but its rollout is proving to be a more limited affair than many anticipated. While hailed by some as a potential game-changer offering a different vaccine technology, its delayed arrival and narrower target market raise important questions about its overall impact on the pandemic response.
A Different Approach: Protein Subunit Technology
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax utilizes a protein subunit technology. This method uses purified fragments of the virus to trigger an immune response, offering a potentially more familiar approach for those hesitant about newer vaccine technologies. This traditional approach may appeal to a segment of the population who have been reluctant to receive mRNA vaccines, potentially boosting overall vaccination rates. However, the perception of familiarity needs to be weighed against the vaccine's late entry into a market already saturated with other options.
Limited Rollout: Why the Cautious Approach?
The limited rollout is attributed to several factors. Firstly, the vaccine's development and approval process faced significant delays, resulting in its arrival long after other vaccines had established themselves. Secondly, manufacturing capacity constraints have initially restricted the vaccine's availability. While Novavax has pledged to increase production, the initial supply is insufficient to make a significant dent in global vaccination efforts. Finally, the FDA's conditional approval itself is a reflection of a cautious approach, given the existing vaccine landscape. The agency is carefully monitoring its efficacy and safety profile in the real world.
Who is the Target Audience?
Currently, the Novavax vaccine is primarily targeted towards adults who have not yet received a COVID-19 vaccine, or those who prefer a protein subunit vaccine. While it's authorized for use in individuals 18 years and older, its limited availability means that it's unlikely to become a widespread choice immediately. This targeted approach is a strategic move by health authorities, focusing resources on those populations most likely to benefit.
Efficacy and Safety Concerns:
The FDA's conditional approval hinged on data demonstrating the vaccine's efficacy and safety. While clinical trials showed it to be effective in preventing COVID-19, its efficacy might be slightly lower compared to some other available vaccines. Furthermore, post-market surveillance will be crucial to monitor for any rare or unexpected side effects. The agency will continue to collect data to ensure the vaccine's long-term safety and effectiveness.
The Future of Novavax:
The Novavax vaccine's future remains uncertain. Its success will depend on factors such as increased manufacturing capacity, effective communication campaigns targeting the intended population, and continued monitoring of its safety profile. While it may not revolutionize the COVID-19 vaccination landscape, it offers a valuable alternative for specific populations, potentially contributing to higher overall vaccination rates and broader protection against the virus. The long-term impact remains to be seen, but the vaccine represents a significant addition to the global fight against the pandemic.
Call to Action: Consult your healthcare provider to discuss whether the Novavax vaccine is the right choice for you. Stay informed about the latest updates on COVID-19 vaccines and vaccination recommendations from reliable sources like the and .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine's Limited Rollout. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 J D Vance On Bidens Cancer Diagnosis Questions Of Presidential Fitness
May 21, 2025
J D Vance On Bidens Cancer Diagnosis Questions Of Presidential Fitness
May 21, 2025 -
 Tuesday Night Storm Forecast Low Probability Of Intense Weather
May 21, 2025
Tuesday Night Storm Forecast Low Probability Of Intense Weather
May 21, 2025 -
 A Quieter Wes Anderson Exploring The Subtleties Of The Phoenician Scheme
May 21, 2025
A Quieter Wes Anderson Exploring The Subtleties Of The Phoenician Scheme
May 21, 2025 -
 Taylor Jenkins Reids Publishing Dominance Analyzing Her Career Trajectory
May 21, 2025
Taylor Jenkins Reids Publishing Dominance Analyzing Her Career Trajectory
May 21, 2025 -
 De Generes Grief A Heartbreaking Update Following Family Death
May 21, 2025
De Generes Grief A Heartbreaking Update Following Family Death
May 21, 2025
