Conditional FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval for Novavax COVID-19 Vaccine: What You Need to Know
The landscape of COVID-19 vaccines has expanded with the Novavax vaccine receiving Conditional FDA approval. This marks a significant development, offering an alternative option for individuals hesitant about mRNA vaccines like Pfizer-BioNTech and Moderna. But what exactly does this mean, and what should you know before considering the Novavax vaccine?
Understanding Conditional FDA Approval:
Unlike full FDA approval, which requires extensive long-term data, conditional approval allows for quicker authorization based on substantial evidence of the vaccine's safety and efficacy. The FDA grants conditional approval when the benefits clearly outweigh the risks, particularly during public health emergencies like the COVID-19 pandemic. This means ongoing monitoring and data collection will continue to assess the vaccine's long-term safety and effectiveness. Learn more about the FDA approval process .
How Does the Novavax Vaccine Work?
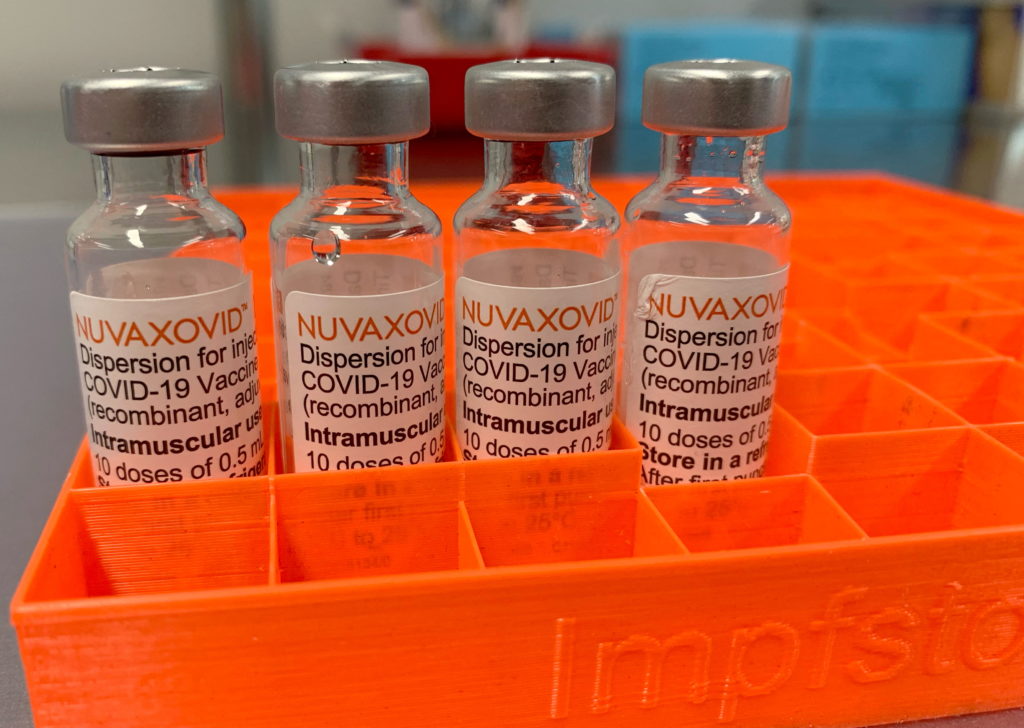
Unlike mRNA vaccines that use messenger RNA to instruct cells to produce a viral protein, the Novavax COVID-19 vaccine, known as Nuvaxovid, uses a more traditional approach. It's a protein subunit vaccine. This means it contains a purified, harmless piece of the SARS-CoV-2 virus (the spike protein) that triggers an immune response. This approach has been used in other vaccines for decades, potentially making it a more familiar and less anxiety-inducing option for some.
Efficacy and Safety:
Clinical trials demonstrated that the Novavax vaccine is highly effective in preventing COVID-19. It showed a significant reduction in symptomatic infections and serious illness. Like all vaccines, side effects are possible, though generally mild and temporary. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and nausea. Serious side effects are rare. For detailed information on reported side effects, consult the official Novavax website or the CDC's resources.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine is authorized for individuals 18 years of age and older. It presents a viable alternative for those who:
- Are hesitant about mRNA vaccines.
- Prefer a more traditional vaccine technology.
- Have specific medical concerns that make mRNA vaccines less suitable.
Addressing Vaccine Hesitancy:
Vaccine hesitancy remains a significant public health challenge. Understanding the different vaccine technologies available and discussing concerns with a healthcare professional can help alleviate fears and promote informed decision-making. The availability of the Novavax vaccine provides an additional tool in the fight against COVID-19, potentially reaching populations previously hesitant to receive a vaccine. Explore resources on vaccine safety and efficacy from reputable organizations like the and -vaccines).
Looking Ahead:
The conditional FDA approval of the Novavax COVID-19 vaccine is a significant step forward in the global fight against the pandemic. Its availability adds another layer of options for individuals and public health strategies, offering a potentially less intimidating alternative for those hesitant about other vaccine types. Continued monitoring of the vaccine’s long-term safety and efficacy is crucial, ensuring its continued role in protecting communities against COVID-19. Consult your doctor to determine if the Novavax vaccine is the right choice for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Helldivers 2 Warbond Drop Master Of Ceremony Arrives May 15th
May 20, 2025
Helldivers 2 Warbond Drop Master Of Ceremony Arrives May 15th
May 20, 2025 -
 New Helldivers 2 Warbonds Masters Of Ceremony Cosmetic Items Available May 15th
May 20, 2025
New Helldivers 2 Warbonds Masters Of Ceremony Cosmetic Items Available May 15th
May 20, 2025 -
 Medical And Scientific Research A Foundation Of American Strength
May 20, 2025
Medical And Scientific Research A Foundation Of American Strength
May 20, 2025 -
 Balis Tourism Overhaul Stricter Guidelines To Curb Negative Tourist Behavior
May 20, 2025
Balis Tourism Overhaul Stricter Guidelines To Curb Negative Tourist Behavior
May 20, 2025 -
 Immigration And Citizenship A Potential Dhs Reality Tv Show
May 20, 2025
Immigration And Citizenship A Potential Dhs Reality Tv Show
May 20, 2025
